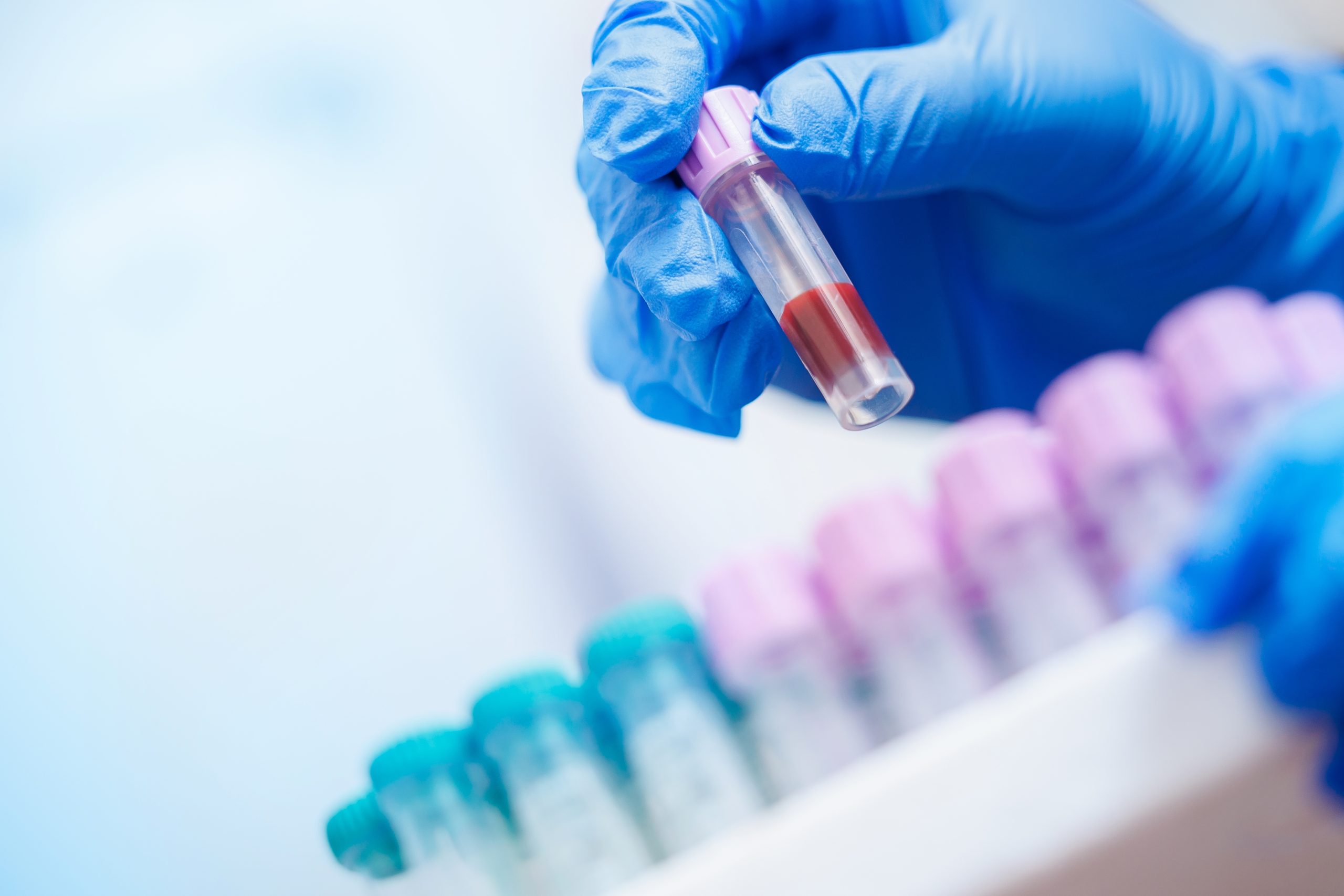
Scientists can now detect cancer in your bloodstream up to three years before you experience any symptoms.
Story Overview
- FDA approved the first blood-based cancer detection tests in 2020, marking a paradigm shift from invasive tissue biopsies to simple blood draws
- Johns Hopkins research indicates these “liquid biopsies” can identify cancer-related DNA changes years before clinical diagnosis
- Current tests excel at monitoring treatment response and detecting recurrence, but widespread screening applications require further validation
- The technology builds on decades of cancer genetics research, from the 1928 Pap smear breakthrough to modern genomic profiling
The Promise of Detecting the Undetectable
The 2020 FDA approval of blood-based cancer detection tests represents the culmination of nearly a century of cancer research evolution. These liquid biopsies work by identifying tumor DNA that sheds into the bloodstream, eliminating the need for invasive tissue sampling. Unlike traditional methods requiring symptoms or visible tumors, these tests can potentially catch cancer at its earliest stages.
The technology leverages advanced genomic analysis to scan for cancer-related genetic changes. Tests like MSK-IMPACT analyze 468 cancer-related genes, while FoundationOne CDx evaluates 324 genes known to fuel cancer growth. This precision enables targeted treatment selection based on individual tumor genetics rather than cancer type alone.
Watch:
From Laboratory Discovery to Clinical Reality
The foundation for liquid biopsies traces back to landmark discoveries in cancer research. George Papanicolaou’s 1928 Pap smear demonstrated that abnormal cells could be detected before becoming cancerous. The 1960 discovery of the Philadelphia chromosome established the first genetic marker for a specific cancer type, chronic myelogenous leukemia.
These early breakthroughs led to the 1986 cloning of the HER2 oncogene, which overexpresses in 20-25% of breast cancers. This discovery enabled the development of trastuzumab, the first targeted cancer therapy approved by the FDA in 1998. By 2017, comprehensive genomic profiling tests became FDA-cleared, setting the stage for blood-based detection technologies.
Current Clinical Applications and Limitations
Today’s liquid biopsy tests demonstrate proven effectiveness in specific clinical scenarios. They excel at monitoring treatment response in patients with known cancer diagnoses and detecting recurrence after treatment completion. The NCI-MATCH clinical trial, launched in 2015, uses these tests to match patients with targeted therapies based on tumor genetics regardless of cancer type.
However, the transition from promising research to widespread screening faces significant challenges. Test standardization remains inconsistent across different platforms and manufacturers. Healthcare providers require specialized training to interpret results and counsel patients appropriately.
The Road Ahead for Cancer Detection
The medical community recognizes liquid biopsies as a transformative advancement while acknowledging implementation hurdles. Current evidence strongly supports their use for treatment monitoring and recurrence detection. Broader application for asymptomatic population screening awaits additional clinical validation and regulatory clarity from the FDA.
If validated for widespread screening, these tests could fundamentally shift cancer care from reactive treatment to proactive prevention. The potential to detect cancer three years before symptoms appear offers unprecedented opportunities for early intervention when treatments are most effective. Need a doctor right now? Connect instantly through My Healthy Doc
Sources:
National Cancer Institute: 250 Years of Cancer Research Milestones
American Association for Cancer Research: Landmarks in Cancer Research 1907-1960
Baptist Health: Could a Simple Blood Test Detect Cancer Years Before Symptoms