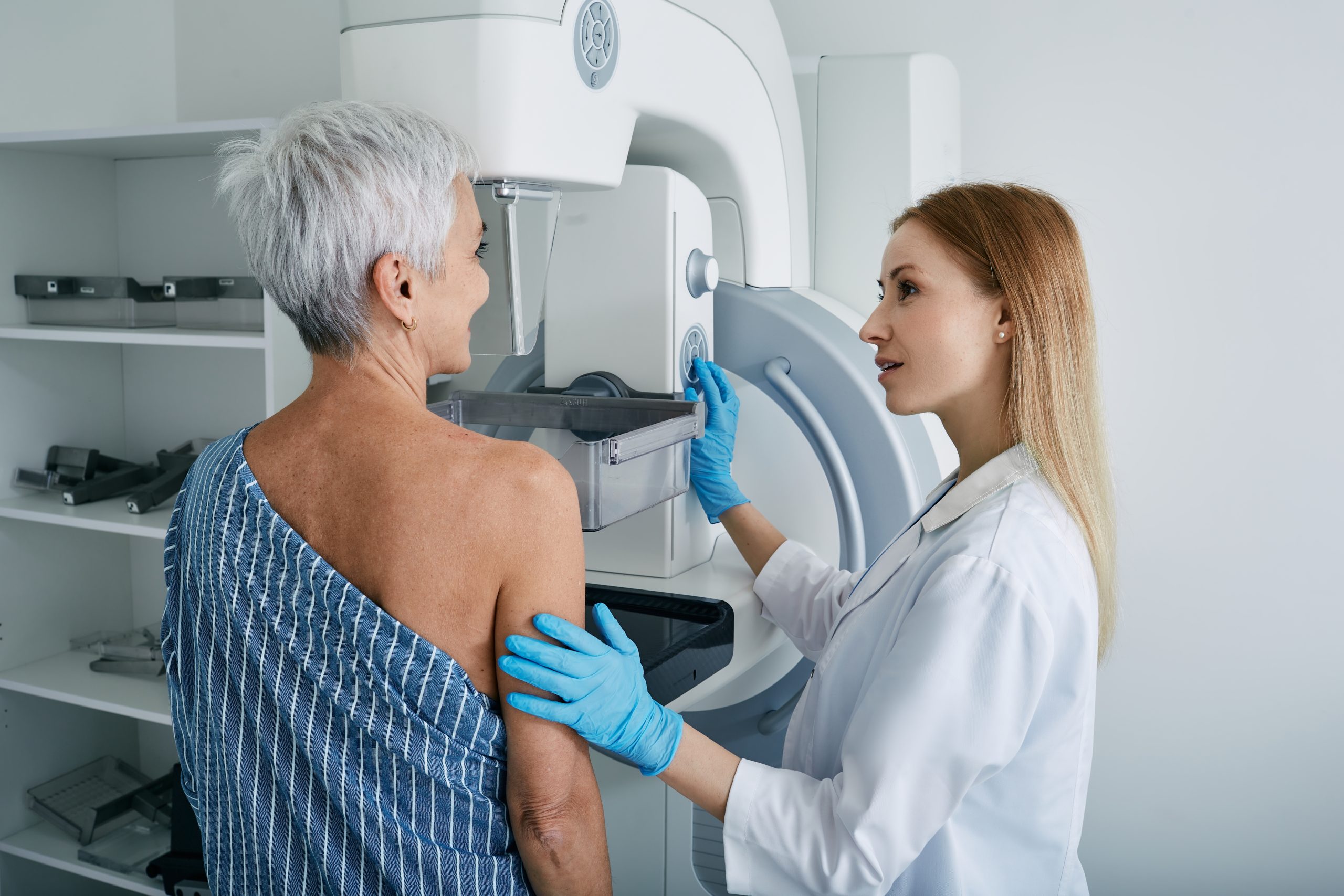
Revolutionary AI technology is transforming breast cancer screening by predicting a woman’s five-year cancer risk with 2.2 times greater accuracy than traditional methods.
Story Highlights
- Washington University’s AI software receives FDA Breakthrough Device designation for superior breast cancer risk prediction
- New technology analyzes mammograms to predict five-year cancer risk 2.2 times more accurately than questionnaire-based methods
- Hologic’s AI system successfully flagged 32% of breast cancers initially missed by radiologists in 7,500 screening exams
- $16 million PRISM trial launches across five states to validate AI-assisted mammogram reading in real-world settings
FDA Breakthrough Accelerates Cancer Prevention Innovation
Washington University School of Medicine researchers developed groundbreaking AI software that earned FDA Breakthrough Device designation for its ability to predict breast cancer risk using only mammogram images and patient age. Licensed to startup Prognosia Inc., this technology represents a fundamental shift from subjective questionnaire-based risk models to objective AI-driven analysis. The software works with both 2D and synthetic 3D mammograms, enabling seamless integration into existing screening infrastructure worldwide without requiring additional patient data or imaging procedures.
AI Detection Catches What Human Eyes Miss
Hologic’s Genius AI Detection system demonstrated remarkable capabilities in a 2025 study of 7,500 screening exams, successfully flagging 32% of breast cancer cases that radiologists initially interpreted as negative. The AI system also detected 90% of known cancer cases, showcasing its precision in identifying malignancies. Dr. Manisha Bahl from Mass General Brigham noted the technology’s potential to “redefine detection” by providing precise localization of suspicious areas, enabling earlier intervention and improved patient outcomes through enhanced accuracy.
A smarter way to screen for breast cancer is emerging: https://t.co/bJmtsgVKT9
— Ken Gusler (@kgusler) January 4, 2026
Massive Clinical Trial Tests Real-World Effectiveness
The $16 million PRISM trial, funded by the Patient-Centered Outcomes Research Institute, represents the first large-scale randomized U.S. study examining AI’s role in mammogram interpretation. Led by UC Davis Health and UCLA Health, the trial spans California, Florida, Massachusetts, Washington, and Wisconsin, comparing AI-assisted readings to standard radiologist interpretations. Diana Miglioretti from UC Davis emphasized this groundbreaking research will provide crucial evidence on AI’s real-world effectiveness in reducing false positives and improving detection rates.
American Innovation Leads Global Healthcare Revolution
These advances position American medical institutions at the forefront of AI-driven healthcare innovation, potentially reducing healthcare costs while improving patient outcomes. The technology addresses critical radiologist shortages while maintaining the human expertise essential for patient care. Graham Colditz from Washington University highlighted the global potential, noting the AI can be “available worldwide via existing mammograms,” democratizing access to advanced screening capabilities without requiring expensive infrastructure upgrades or additional medical personnel training.
Watch:
This breakthrough represents American ingenuity solving real healthcare challenges through technology that enhances rather than replaces medical professionals. The combination of rigorous clinical trials, FDA oversight, and practical implementation ensures these innovations will benefit patients while maintaining the highest safety standards that Americans deserve from their healthcare system.
Your instant doctor companion – online 24 hours a day.
Sources:
AI-based breast cancer risk technology receives FDA Breakthrough Device designation
Artificial Intelligence in Breast Cancer Screening
UC Davis Health to co-lead $16 million study examining AI’s role in reading mammograms